Top Legislative Priorities Before Election Day
When Congress returns from its extended 7-week summer recess on September 6th, they will have just 17 workdays before adjourning for a pre-election recess. Some refer to this as the "lightning round of legislating," with a lot of work to be done and not much time to do it.
Childhood Cancer STAR Act
The Childhood Cancer STAR Act will officially record its 250th cosponsor when the House returns from Recess -- but we are running out of time to receive a hearing and secure passage of this bipartisan, comprehensive childhood cancer legislation.
In order for the House Energy & Commerce Committee to consider this bill as one of their last priorities of this Congress, they need to hear from constituents about why this life-saving legislation is so important.
Please take a moment to fill out our Action Alert and tell Congress our kids can't afford to wait until the next Congress for action.
NIH Funding
Congress is tasked with deciding how much funding to provide the National Institutes of Health - and the National Cancer Institute - for 2017, as part of the larger appropriations process. As reported on our blog, a House Appropriations committee approved a spending bill earlier this summer that would increase NIH funding by $1.25 billion, while Senate appropriators would increase the NIH budget by $2 billion.
We are urging advocates to write to their Senators and Representatives and ask them to support the higher Senate number. It is imperative that the NIH and NCI continue to work for new treatments and explore the promise of immunotherapy. While we recognize that Congress faces difficult budget decisions, we also believe that Congress must further prioritize cancer research.
Take action now and tell Congress why each additional research dollar matters for children with cancer.
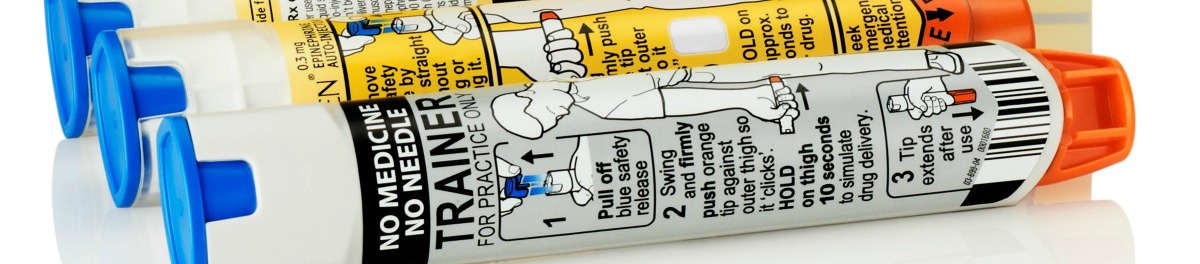
Take Action: Pediatric Cancer Families Feel Impact of EpiPen Price Gouging
As many kids go through cancer treatment, one of the supportive care drugs they are provided is epinephrine in an auto-injector - or epi-pen. Epinephrine is a form of adrenalin and is primarily used to treat allergic reactions to food, insects, or other substances. But it's also provided to kids in cancer treatment in case of a reaction to the drugs they're being given.
Over the past several yeras, the price of epi-pens has increased by some 400 percent. With the recall of a competing product, the manufacturer of the epi-pen, Mylan Pharmaceuticals, faces little market pressure and used the opportunity to escalate the price. Since the cost of providing epi-pens to parents and patients may often be out-of-pocket, these price increases are especially troubling and represent an added burden to families who are already struggling with the treatment of a devastating disease.
Patients from a wide variety of communities are protesting the price-gouging and calling on Congress to intervene. On behalf of the pediatric cancer community, we encourage advocates to join in this appeal by signing on to our petition urging their representatives to speak up for patients and put a lid on profits over patients.
Sign our petition urging Congress to take action:
https://actionsprout.io/FC5265
DC Event Line-Up: Childhood Cancer Awareness Month
Webinar on Psychosocial Impact of Childhood Cancer
September 1
The NCI is hosting an hour-long webinar on the psychosocial and social impacts of pediatric and adolescent cancers -- and how research and therapies are being created to address the unique needs of patients, siblings, parents, friends and others. Use hashtag #AskCCR to pose questions in advance on Twitter, and watch the webinar here.
Panel Discussion: Translating Discovery into Cures for Children with Cancer
September 8, 12:00pm-1:30pm, B-340 Rayburn House Office Building
This panel discussion, co-hosted by the Alliance for Childhood Cancer and the American Cancer Society Cancer Action Network, will examine the current state of pediatric cancer research and the unique challenges to developing new drugs to treat children with cancer.
See invite for RSVP information.
CureFest 2016
September 17-18
CureFest is a festival held on the National Mall that brings thousands of childhood cancer advocates and supporters together with the general public and tourists to raise awareness about pediatric cancer. The event features informational booths, games and activities, live entertainment, and an awareness walk. For the first time this year, all events associated with CureFest are free of charge, although participants are still asked to register in advance.
Be sure to stop by the Legislative Initiatives table at CureFest! There will be educational mini-sessions on current legislation and action steps you can take to make an impact.
Rally for Medical Research Hill Day
September 22
This annual event brings together nearly 300 national organizations, including the Children's Cause, to ask Congress to make funding for the National Institutes of Health (NIH) a national priority. Learn more.
Congressional Childhood Cancer Caucus Summit
September 23, 9-11am, Capitol Hill
Details on the 7th Annual Childhood Cancer Summit will be posted here when available.
Alliance Art Show and Luncheon
September 23, 11-1pm, Capitol Hill
Details will be shared when available.
Help us gear up for Childhood Cancer Awareness Month by strengthening our story bank. By sharing your story with us today, you empower us to have a much greater impact this September:
Recommended Reading: Quick Links
- Children's Cause Responds to FDA's Status Report on PREA: The Food and Drug Administration released a status report in July on the Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA). Several Alliance for Childhood Cancer recommendations for improving PREA made it into the report, including an amendment that would require certain drugs developed for adult cancer indications to be evaluated for a pediatric cancer indication when there is evidence the drug affects specific molecular targets and/or mechanisms shared between adult and pediatric cancers. Read full letter. (CCCA, 8/18/16)
- CAC2 Summit in Philadelphia: The Coalition Against Childhood Cancer held its 4th annual meeting and summit earlier this summer in Philadelphia -- its "largest and most comprehensive educational experience yet." The Children's Hospital of Philadelphia wrote up a terrific overview of the event: Childhood Cancer Advocates Find Strength in Numbers. (CHOP, August 2016)
- Book Recommendation - Cooking for Kids with Cancer: To mark "Book Lovers' Day" earlier this month, we reviewed a new book by Chef Ryan Callahan, Cooking for Kids with Cancer. This book focuses on the food challenges facing children undergoing cancer treatment, and we include an excerpt and a sample recipe on our blog. Read more. (CCCA Blog, 8/9/16)
New York Reception: November 3
Mark your calendars for the annual New York Reception for the Children's Cause for Cancer Advocacy on November 3. This year's event will take place at the James Burden Mansion in New York City from 7:00-9:30pm.
Proceeds benefit CCCA's mission of advocating for less toxic treatments, federal funding for research, and better follow-up care for the unique needs of childhood cancer patients and survivors.
Look for email invitation to come in September. If you have questions in the meantime, contact Julie at jtaylor@childrenscause.org.